Convert Sce To Ag Agcl
Convert Sce To Ag Agcl. The standard potential of the ag/agcl electrode has been reported at temperatures exceeding \(\si{100}{\degreecelsius}\). Up to $2.56 cash back get the detailed answer:
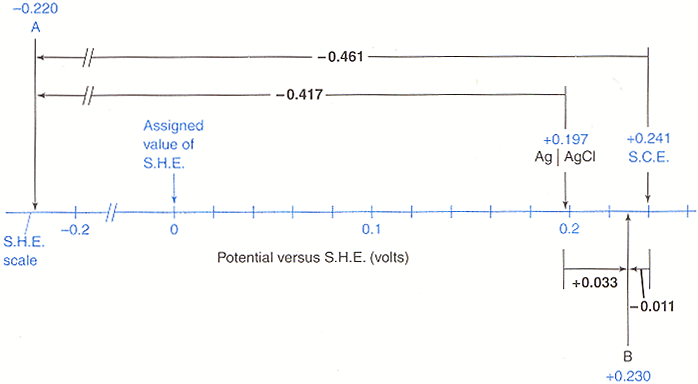
Primary and secondary reference electrodes. Next, select the reference electrode you. We couldn't find a conversion between moles agcl and molecule do a quick conversion:
Conversion Of Relative Reference Electrode Potentials Into.
Enter the recorded voltage in mv and select the reference electrode used to record it. V (on sce, sat.) to the she scale. The standard potential of the ag/agcl electrode has been reported at temperatures exceeding \(\si{100}{\degreecelsius}\).
Ag/Agcl But In Order To Calculate The;
This little snippet can convert electrode potential scales between the most common reference electrodes used in corrosion research. Fundamentals and applications, a j bard and l r faulkner, john. So in this question, were using tabulated electro potentials to calculate delta g not of reaction.
Our Conversion Constants For The Ape And Anes Versus She , , , Are Indeed In The Range Of The +560 Mv Most Often Proposed As The Recalculation Constant For Various ‘Ag + /Ag’ Electrodes.
The measurement of electrode potentials requires the use of a reference electrode. For sake of clarity, considering the different ph of the solutions, all potentials are referred to reversible hydrogen electrode (rhe) according to the following formula: Reference electrodes must exhibit a stable potential value under the specified polarization conditions;
This Calculator Can Be Used To Convert Potentials Recorded Using One Reference Electrode Into Potentials Reported Versus Another, More Commonly Accepted, Reference Electrode.
We couldn't find a conversion between moles agcl and molecule do a quick conversion: E (vs rhe) = e (vs sce) + 0.244 + 0.059ph, with 0.244 the potential (in volt) of saturated calomel electrode (sce) with respect to standard hydrogen. Primary and secondary reference electrodes.
Ag/Agcl Electrodes Can Be Used Up To 100°C (Depending On The Materials Used To Make The Electrode), And Are Commercially Available From Many Companies.
It has a reputation of being very stable and robust. So, the short answer is you should subtract 45 mv from readings obtained with an ag/agcl,sat'd nacl reference electrode in order to convert to potential vs. Then calculate the potential of the following cell at 25 c:
Post a Comment for "Convert Sce To Ag Agcl"